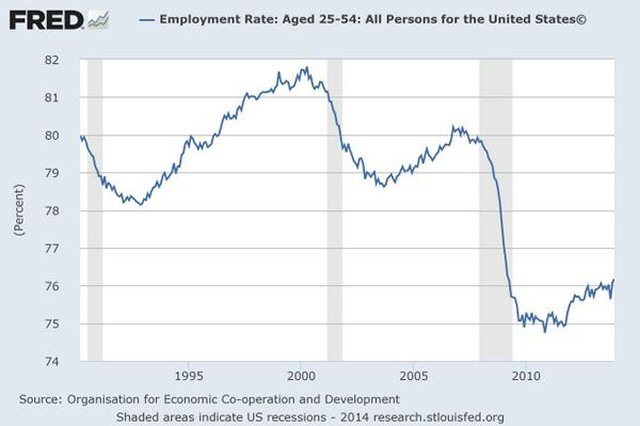
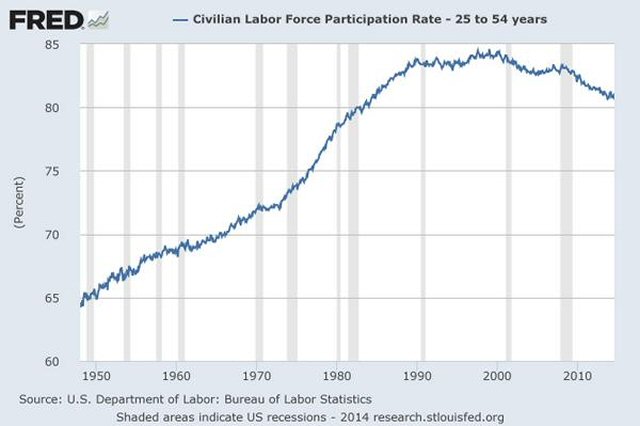
The South–North Water Transfer Project is a multi-decade infrastructure project of the People’s Republic of China to better utilize water resources available to China. This is to be achieved through the South North Water Diversion Project (SNWD). While the main thrust is to divert water from the Yangtze River to the Yellow River and Hai River, other spin-off plans are also loosely included. Amongst these, a controversial plan calling for the capture and diversion of water from Brahmaputra River, located in Yarlung Tsangpo Grand Canyon north of India, has been under study for years. This is because heavily industrialized Northern China has a much lower rainfall and its rivers are running dry. Already the Yellow River has often gone dry in its lower reaches in recent decades and some of the Hai River tributaries almost dried out throughout the year. Supply and demand conditions have often changed more rapidly than the project plans’ ability to accommodate the changes, resulting in much higher costs and reduced benefits.
Project’s conception
The idea for the South–North Water Transfer Project originated from Mao Zedong who said, “Southern water is plentiful, northern water scarce. If at all possible, borrowing some water would be good.” After his comments, the Chinese Water Works Department conducted several studies on the project. After decades of study, the South–North Water Transfer Project settled on three different proposals for routes: The western route is in the western headwaters of the rivers where the Yangtze River and the Yellow River are closest to one another; the central route is from the upper reaches of the Han River (a tributary of Yangtze River) to Beijing and Tianjin; and the eastern route uses the course of the Grand Canal. This project will divert 44.8 billion cubic meters/year of water from South to North.
Western route
The western route, called the Big Western Line, is to divert water from the headwater of the Yangtze River into the headwater of the Yellow River. In order to move the water through the drainage divide between these rivers, huge dams and long tunnels are needed to be built to cross the Qinghai-Tibetan Plateau and Western Yunnan Plateaus. In addition, about 200 billion cubic meters (200 cubic km) of water will be diverted from the upstream sections of six rivers in southwestern China annually, including the Mekong, the Yarlung Zangbo and the Salween, to the dry areas of northern China through a system of reservoirs, tunnels and natural rivers. The feasibility of this route is still under study and this project won’t start in the near future. Environmentalists have raised concerns about potential flooding that could result.
Central route
The central route is from Danjiangkou Reservoir on the Han River, a tributary of the Yangtse River, to Beijing. This route is built on the North China Plain and, once the Yellow River has been crossed, water can flow all the way to Beijing by gravity. The main engineering challenge is to build a tunnel under the Yellow River. Construction on the central route began in 2004. In 2008 the 307 km-long Northern stretch of the central route was completed at a cost of US$2 billion. Water in that stretch of the canal does not yet come from the Han River, but from various reservoirs in Hebei Province south of Beijing. Farmers and industries in Hebei had to cut back water consumption to allow for water to be transferred to Beijing.
The whole project was expected to be completed around 2010. This has recently been set back to 2014 to allow for more environmental protections to be built. A problem is the influence on the Han River, where ~1/3 of the water is diverted. One long-term consideration is to build another canal to divert water from the Three Gorges Dam to Danjiangkou Reservoir. Another major difficulty is the resettlement of ~330,000 persons around Danjiangkou Reservoir and along the route. On October 18, 2009, Chinese officials began to relocate residents from the areas of the Hubei and Henan provinces that will be affected by the reservoir.
Eastern route
The Grand Canal is currently being upgraded. Water from the Yangtze River will be drawn into the canal in Jiangdu, where a giant 400 m³/s (12.6 Billion m3/year if operated continuously) pumping station was built already in the 1980s, and is then fed uphill by pumping stations along the Grand Canal and through a tunnel under the Yellow River, from where it can flow downhill to reservoirs near Tianjin. Construction on the Eastern Route officially began on December 27, 2002, and water is supposed to reach Tianjin by 2012. However, water pollution has affected the viability of this project.
The original plan that began in the 1950s and 1960s called for diverting the Nu (Salween), the Lancang (Mekong), the Tongtian, the Yalong, and the Dadu Rivers into the upstream Yellow River. The project was considered too immense and costly to be undertaken at the time. At the present, armed with new technology, feasibility studies have been conducted with the plan of connecting the three latter rivers (the Tongtian, the Yalong, and the Dadu, rivers that flow entirely within the borders of China) and diverting them into the Yellow River. The Tongtian diversion line would be 289 km in length, the Yalong 131 km, and the Dadu 30 km.
Project controversy
Since the introduction of the project, it has created widespread controversy. Opponents to the project object to it on the grounds that it is a waste of resources, it could create a large number of migrant people, it could waste massive amounts of water through evaporation and pollution, the project’s huge cost would make the water prohibitively expensive for consumers, the dry season could cause the Yangtze River to suffer from water shortages, it would be detrimental to the Yangtze River’s transportation, and it could cause an environmental disaster. Additionally, some villagers being relocated for the Central route claim they were forced to sign relocation agreements. Government officials and defenders of the project claim the Yangtze River has a plentiful supply of water, with 96% of the water currently flowing into the Pacific Ocean. They argue that transferring one portion to the poorly irrigated areas of the North could solve the North’s water scarcity issue.
Water resources of the People’s Republic of China
The water resources of China are affected by both severe water quantity shortages and severe water quality pollution. A growing population and rapid economic development as well as lax environmental oversight have increased water demand and pollution. China has responded by measures such as rapidly building out the water infrastructure and increasing regulation as well as exploring a number of further technological solutions.
Supply
China’s water resources include 2,711.5 cubic kilometers of mean annual run-off in its rivers and 828.8 cubic kilometers of groundwater recharge. As pumping water draws water from nearby rivers, the total available resource is less than the sum of surface and groundwater, and thus is only 2,821.4 cubic kilometers. 80% of these resources are in the South of China.
Demand
Total water withdrawals were estimated at 554 cubic kilometers in 2005, or about 20% of renewable resources. Demand is from the following sectors:
65% agriculture
23% industry
12% domestic
In 2006 626,000 square kilometers were irrigated.
Water Balance
Over-extraction of groundwater and falling water tables are big problems in China, particularly in the north. According to the Ministry of Construction, preliminary statistics show that there are more than 160 areas nationwide where groundwater has been over-exploited with an average annual groundwater depletion of more than 10 billion cubic meters. As a result, more than 60,000 square kilometers of ground surface have sunk with more than 50 cities suffering from serious land subsidence. Flooding also still is a major problem.
In a Xinhua article from 2002, Chinese experts warned of future or current water shortages. Water resource usage was expected to peak in 2030 when the population peaks. Areas north of the Yangtze River were particularly affected with 80.9% of Chinese water resources being south of the river. Northern China had used 10,000-year-old aquifers which had resulted in ground cracking and subsidence in some regions.
A 2005 article in China Daily stated that out of 514 rivers surveyed in 2000, 60 were dry. Water volume in lakes had decreased by 14%. Many wetlands had decreased in size.
Jared Diamond stated in his 2005 book Collapse that, in the past 50 years, exploitation in the form of dams and other irrigation infrastructure have all but halted the Yellow River’s natural course, threatening to dry up the entire river valley. The cessation of river flows, or flow stoppages, has surged since the 1980s because of increased water usage and waste. In 1997, the lower Yellow River did not flow 230 days out of the year, an increase of over 2000% since 1988. Increased erosion and sedimentation, especially on the Loess Plateau, has made the river much less navigable by ship.
For the 2008 Summer Olympics, China diverted water from Hebei and Shanxi provinces, areas already beset by drought and dramatic water shortages, to Beijing. In July 2008, the head of the Beijing Water Authority Bi Xiaogang denied that the Olympics would increase water consumption by a large amount. However, previously he and other local officials said that Beijing would divert up to 400 million cubic meters of water from Hebei for the Games with water-diversion facilities and pipes being built to pump water from four reservoirs in Hebei. Around Baoding city alone, a mostly rural area, 31,000 residents lost land and their homes due to a water transfer project; many more have been displaced throughout Hebei. According to an August 24, 2008 report by the UK’s Times, much of the infrastructure intended for the water diversion scheme was left half-constructed or unused when Beijing officials realized that water demand estimates had been far too high. The number of tourists attending the Beijing games was lower than expected, and many migrant workers, ethnic minorities, and political dissidents had left the city as a result of intimidation or official requests. Nevertheless, the Hebei area had already been sucked dry to fill a number of large reservoirs, leading to drought and agricultural losses.
Water Transfers
Large-scale water transfers have long been advocated by Chinese planners as a solution to the country’s water woes. The South-North Water Transfer Project is being developed primarily to divert water from the Yangtze River to the Yellow River and Beijing.
The development or diversion of major trans-boundary rivers originating in China, such as the Brahmaputra River and the Mekong River, could be a source on tension with China’s neighbors. For example, after building two dams upstream, China is building at least three more on the Mekong, inflaming passions in Vietnam, Laos, Cambodia and Thailand. In a book titled “Tibet’s Waters Will Save China” a group of Chinese ex-officials have championed the northward rerouting of the waters of the Brahmaputra as an important lifeline for China in a future phase of South-North Water Transfer Project. Such a diversion could fuel tension with India and Bangladesh, if no prior agreement would be reached on sharing the river’s water.
Sea Water Desalination
Due to the water problems, as well as for future exports, China is building up its desalination technological abilities and plans to create an indigenous industry. Some cities have introduced extensive water conservation and recycling programs and technologies.
Water Quality
The quality of groundwater or surface water is a major problem in China, be it because of man-made water pollution or natural contamination.
Deterioration of drinking water quality continues to be a major problem in China. Continuous emissions from manufacturing is the largest contributor to lowered drinking quality across the People’s Republic, but introduction of poorly treated sewage, industrial spills, and extensive use of agricultural fertilizers and pesticides have proven to be major contributors as well. Furthermore, these water quality issues couple with seasonal scarcity of water to spark endemic water shortages, which frequently affect millions of people to some extent.
According to China’s State Environmental Protection Administration (SEPA) in 2006, 60% of the country’s rivers suffer from pollution to such an extent that they cannot be safely used as drinking water sources. According to the 2008 State of the Environment Report by the Ministry of Environmental Protection, the successor agency of SEPA, pollution of specific rivers is as follows:
The Pearl River and the Yangtze River had “good water quality”; the Songhua River was “slightly polluted” (it was “moderately polluted” in 2006); the Liaohe River, the Huaihe River, and the Yellow River were “moderately polluted” (another translation says they “had poor water quality”); and the Haihe River which flows through Beijing and Tianjin was “badly polluted”.
A 2006 article by the Chinese Embassy in the UK stated that approximately 300 million nationwide have no access to clean water. Almost 90% of underground water in cities are affected by pollution and as well as 70% of China’s rivers and lakes.
A 2007 article in China Daily stated that large scale use of pesticides and fertilizers from agriculture also contribute to water pollution.
A 2008 report about the Yellow River argued that severe pollution caused by factory discharges and sewage from fast-expanding cities has made one-third of the river unusable even for agricultural or industrial use. The report, based on data taken last year, covered more than 8,384 miles of the river, one of the longest waterways in the world, and its tributaries. The Yellow River Conservancy Committee, which surveyed more than 8,384 miles of the river in 2007, said 33.8% of the river system registered worse than level five. According to criteria used by the UN Environment Program, level five is unfit for drinking, aquaculture, industrial use and even agriculture. The report said waste and sewage discharged into the system last year totaled 4.29bn tons. Industry and manufacturing provided 70% of the discharge into the river, with households accounting for 23% and just over 6% coming from other sources.
There have been a high number of river pollution incidents in recent years in China, such as drinking water source pollution by algae in the Tai Lake, Wuxi in May 2007. There was a “bloom of blue-green algae that gave off a rotten smell” shutting off the main source of drinking water supply to 5.8 million people. By September 2007, the city had closed or given notice to close more than 1,340 polluting factories. The city ordered the rest to clean up by June or be permanently shut down. The closing of the factories resulted in a 15% reduction of local GDP. The severe pollution had been known for many years, but factories had been allowed to continue to operate until the crisis erupted.
The 2005 Jilin chemical plant explosions in Jilin City caused a large discharge of nitrobenzene into the Songhua River. Levels of the carcinogen were so high that the entire water supply to Harbin city (pop 3.8M) was cut off for five days between November 21, 2005 and November 26, 2005, though it was only on November 23 that officials admitted that a severe pollution incident was the reason for the cutoff.
Chinese environmental activist and journalist Ma Jun warned in 2006 that China is facing a water crisis that includes water shortages, water pollution and a deterioration in water quality. Ma argued that 400 out of 600 cities in China are facing water shortages to varying degrees, including 30 out of the 32 largest cities. Furthermore, Ma argued, discharges of waste water have increased continually over the years 2001-2006, and that that 300 million peasants’ drinking water is not safe. He warned: “In the north, due to the drying up of the surface water, the underground water has been over-extracted. The water shortage in the north could have drastic affects because almost half of China’s population lives on only 15 percent of its water. The situation is not sustainable. Though the south has abundant water, there is a lack of clean water due to serious water pollution. Even water-abundant deltas like the Yangtze and the Pearl River suffer from water shortages.”
According to an article in the Guardian, in 2005, deputy minister Qiu Baoxing stated that more than 100 out of the 660 cities had extreme water shortages. Pan Yue, deputy director of the State Environment Protection Agency, warned that economic growth was unsustainable due to the water problems. In 2004 the World Bank warned that the scarcity of the resource would lead to “a fight between rural interests, urban interests and industrial interests on who gets water in China.” In April 2005 there were dozens of injuries in Dongyang city, Zhejiang Province, due to clashes over the nearby chemical factories of the Juxi Industrial Park accused of water pollution that harmed crops and led to deformed babies being born. According to the article, a quarter of the population lacked clean drinking water and less than a third of the waste was treated. China is expected to face worsening water shortages until 2030 when the population peaks.
According to a 2007 report by the World Bank, the pollution scandals demonstrate that, if not immediately and effectively controlled, pollution releases can spread across the boundaries of administrative jurisdictions, causing “environmental and economic damage as well as public concern and the potential for social unease”. Once an accident has occurred, the impact on the environment and human health becomes more difficult and more costly to control. Therefore, the report recommends prevention of pollution by strict enforcement of appropriate policies and regulations.
Natural Contamination
Large portions of China’s aquifers suffer from arsenic contamination of groundwater. Arsenic poisoning occurs after long-term exposure to contaminated groundwater through drinking. The phenomenon was first detected in China in the 1950s. As water demand grows, wells are being drilled deeper and now frequently tap into arsenic-rich aquifers. As a consequence, arsenic poisoning is rising. To date there have been more than 30,000 cases reported with about 25 million people exposed to dangerously high levels in their drinking water.
According to the WHO over 26 million people in China suffer from dental fluorosis (weakening of teeth) due to elevated fluoride in their drinking water. In addition, over 1 million cases of skeletal fluorosis (weakening of bones) are thought to be attributable to drinking water. High levels of fluoride occur in groundwater and defluoridation is in many cases unaffordable.
Conservation and Sanitation
Water supply and sanitation in the People’s Republic of China is undergoing a massive transition, while facing numerous challenges – such as rapid urbanization and a widening economic gap between urban and rural areas.
The World Bank in a 2007 report stated that between 1990 and 2005 there have been major financial investments in water infrastructure. While urban water supply coverage increased from 50% to 90%, there are still seasonal water shortages in many cities. Water usage by the growing population has increased but it has decreased by industry causing a stabilization of the overall water usage level. Wastewater treatment of urban wastewater more than tripled from 15% to 52%. Installed wastewater treatment capacity grew much more quickly due to an increasing absolute amount of wastewater. Absolute release of municipal pollutants has decreased slightly since 2000.
According to a 2007 article, the SEPA stated that the water quality in the central drinking water sources for major cities was “mainly good”.
Management
The responsibility for dealing with water is split between several agencies within the government. Water pollution is the responsibility of the environmental authorities, but the water supply itself is managed by the Ministry of Water Resources. Sewage treatment is managed by the Ministry of Construction, but groundwater management falls within the realm of the Ministry of Land and Resources. China grades its water quality in six levels, from Grade I to Grade VI, with Grade VI being the most polluted.
In 2007 Ma Xiancong, a researcher at the Chinese Academy of Social Sciences Institute of Law, identified the following areas where the government failed to act, or tacitly consented, approved or actively took part and so created a worse situation: land appropriation, pollution, excessive mining and the failure to carry out environmental impact assessments. An example of this emerged in 2006, when the State Environmental Protection Administration revealed over a dozen hydroelectric projects that had broken the Environmental Impact Assessment Law.
In 2005 experts warned that China must use Integrated Water Resources Management in order to achieve sustainable development.
From Wikipedia, the free encyclopedia